Unveiling the Secrets of Fluorine: A Journey into the Chemistry of an Enigmatic Element

Fluorine: The Lone Ranger of the Halogens
4.3 out of 5
| Language | : | English |
| File size | : | 12351 KB |
| Screen Reader | : | Supported |
| Print length | : | 144 pages |
Fluorine, the lightest and most reactive element in the halogen group, stands out as a enigmatic and versatile substance in the world of chemistry. Its unique properties, exceptional bonding capabilities, and myriad applications make it a captivating subject of scientific exploration.
This article will embark on a comprehensive journey into the chemistry of fluorine, unraveling its remarkable characteristics, exploring its diverse compounds, and highlighting its significance in various industries. Join us as we delve into the fascinating realm of this extraordinary element.
Atomic Anatomy: Fluorine's Inner Workings
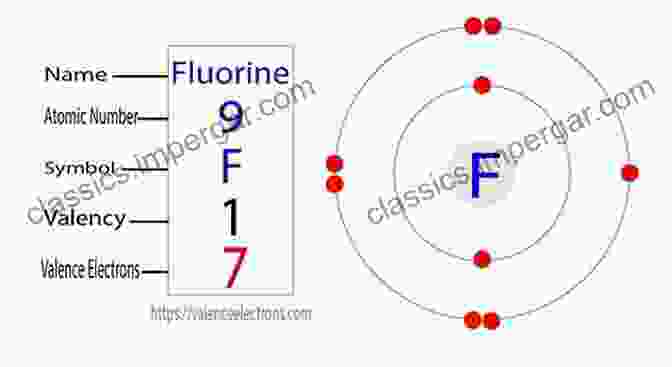
Fluorine, the 17th element on the periodic table, is a highly electronegative and reactive nonmetal. Its atomic number of 9 indicates nine protons and nine electrons, with the outermost electron occupying a lone pair.
Fluorine atoms are exceptionally small, with a covalent radius of just 0.72 angstroms. This diminutive size allows fluorine to form compact and stable bonds with other elements, contributing to the high stability of its compounds.
Chemical Character: Fluorine's Reactive Disposition
Fluorine is renowned for its exceptional reactivity, readily forming compounds with almost every other element except for the noble gases. This reactivity stems from its strong electronegativity, which drives its tendency to attract electrons from other atoms.
Fluorine's high reactivity also manifests in its low ionization energy and high electron affinity. These properties make fluorine an excellent oxidizing agent, capable of transferring electrons to other species and facilitating chemical reactions.
Fluorine's Bonding Prowess: A Versatile Chemical Partner
Fluorine's exceptional reactivity extends to its bonding capabilities, forming diverse and stable compounds with a wide range of elements.
- Ionic Bonds: Fluorine's high electronegativity enables it to form strong ionic bonds with electropositive metals, resulting in ionic compounds such as sodium fluoride (NaF) and calcium fluoride (CaF2).
- Covalent Bonds: Fluorine can also participate in covalent bond formation, sharing electrons with other nonmetals to create covalent compounds like hydrogen fluoride (HF) and sulfur hexafluoride (SF6).
- Coordinate Bonds: Fluorine's lone pair of electrons can engage in coordinate bond formation, donating electrons to metal ions to form coordination complexes.
Fluorine Compounds: From Toothpaste to Semiconductors
Fluorine's versatility in bonding translates into a vast array of compounds with diverse properties and applications.
- Fluorides in Dentistry: Fluoride compounds, such as sodium fluoride and stannous fluoride, are widely used in toothpaste and mouthwashes to strengthen tooth enamel and prevent dental caries.
- Fluorocarbons in Refrigeration: Fluorocarbons, like chlorofluorocarbons (CFCs) and hydrofluorocarbons (HFCs),were once used extensively as refrigerants, but their environmental impact has led to the development of more sustainable alternatives.
- Fluoropolymers in Non-Stick Coatings: Fluoropolymers, such as polytetrafluoroethylene (PTFE),possess exceptional thermal stability and non-stick properties, making them ideal for non-stick cookware, seals, and gaskets.
- Fluorine in Semiconductors: Fluorine is used in the production of semiconductors, particularly in the etching and doping processes, contributing to the miniaturization and performance enhancement of electronic devices.
- Fluorinated Pharmaceuticals: Fluorine-containing pharmaceuticals, such as fluoxetine (Prozac) and ciprofloxacin (Cipro),exhibit unique properties and therapeutic applications, including antidepressant and antibacterial effects.
Safety Considerations: Handling Fluorine Responsibly
While fluorine offers immense benefits in various fields, it is crucial to handle it with utmost care due to its potential hazards.
Fluorine gas is highly toxic and corrosive, requiring specialized handling and storage facilities. Direct contact with fluorine can cause severe burns and respiratory damage. Fluorine compounds, such as hydrogen fluoride, are also corrosive and can irritate the skin, eyes, and lungs.
Proper ventilation, personal protective equipment, and adherence to safety protocols are essential when working with fluorine and its compounds to minimize potential risks.
: Fluorine's Enduring Legacy
Fluorine, with its exceptional reactivity, remarkable bonding versatility, and diverse applications, continues to captivate chemists and scientists worldwide. From its role in strengthening our teeth to its contribution to the development of modern technologies, fluorine's impact on society is undeniable.
As research into fluorine chemistry progresses, we can anticipate even more innovative and groundbreaking applications for this enigmatic element. Its unique properties and potential hold promise for advancements in fields such as energy storage, catalysis, and materials science.
4.3 out of 5
| Language | : | English |
| File size | : | 12351 KB |
| Screen Reader | : | Supported |
| Print length | : | 144 pages |
Do you want to contribute by writing guest posts on this blog?
Please contact us and send us a resume of previous articles that you have written.
 Book
Book Novel
Novel Page
Page Chapter
Chapter Text
Text Story
Story Genre
Genre Reader
Reader Library
Library Paperback
Paperback E-book
E-book Magazine
Magazine Newspaper
Newspaper Paragraph
Paragraph Sentence
Sentence Bookmark
Bookmark Shelf
Shelf Glossary
Glossary Bibliography
Bibliography Foreword
Foreword Preface
Preface Synopsis
Synopsis Annotation
Annotation Footnote
Footnote Manuscript
Manuscript Scroll
Scroll Codex
Codex Tome
Tome Bestseller
Bestseller Classics
Classics Library card
Library card Narrative
Narrative Biography
Biography Autobiography
Autobiography Memoir
Memoir Reference
Reference Encyclopedia
Encyclopedia Ann Gibbons
Ann Gibbons Angelia Clark
Angelia Clark Andrew P King
Andrew P King Andrew Bridgeford
Andrew Bridgeford Anna Proudfoot
Anna Proudfoot Lucas Fain
Lucas Fain Christopher Kinkaid
Christopher Kinkaid Tiffany Mosher
Tiffany Mosher Angela Myles Beeching
Angela Myles Beeching Ann Anderson
Ann Anderson Jim Grant
Jim Grant Andrew Noone
Andrew Noone Robert Gutman
Robert Gutman Amy Roskelley
Amy Roskelley William C Burton
William C Burton Amine Noum
Amine Noum Zari Ballard
Zari Ballard Andrew Gazdecki
Andrew Gazdecki Anjou Kiernan
Anjou Kiernan Joel Berg
Joel Berg
Light bulbAdvertise smarter! Our strategic ad space ensures maximum exposure. Reserve your spot today!

 Gabriel MistralRediscovering the Photographs of Explorer Carl Lumholtz: A Visual Journey...
Gabriel MistralRediscovering the Photographs of Explorer Carl Lumholtz: A Visual Journey...
 Bret MitchellLake Powell Tales: An Anthology of Adventure on America's Second-Largest Lake
Bret MitchellLake Powell Tales: An Anthology of Adventure on America's Second-Largest Lake Shannon SimmonsFollow ·11.4k
Shannon SimmonsFollow ·11.4k Edgar Allan PoeFollow ·16.1k
Edgar Allan PoeFollow ·16.1k Mark MitchellFollow ·19.8k
Mark MitchellFollow ·19.8k Peter CarterFollow ·15.6k
Peter CarterFollow ·15.6k Boris PasternakFollow ·17.3k
Boris PasternakFollow ·17.3k Timothy WardFollow ·3.1k
Timothy WardFollow ·3.1k Tom HayesFollow ·19.4k
Tom HayesFollow ·19.4k Craig BlairFollow ·4.7k
Craig BlairFollow ·4.7k

 Daniel Knight
Daniel KnightUnlock Financial Literacy: Dive into "Accounting...
Embark on an enlightening journey with...

 Dustin Richardson
Dustin RichardsonThe Intrepid Wanda Jablonski and the Power of Information
In the heart of Nazi-occupied...

 Donald Ward
Donald WardMotion For Justice: Rest My Case - An Electrifying Legal...
Prepare to be enthralled as you...
 Felipe Blair
Felipe BlairLeadership Therapy Inside the Mind of Microsoft: A...
Microsoft, a global technology titan, has...

 Voltaire
VoltaireUnlock The Flow State: Boost Your Creativity In Business...
The flow state, also known as...
4.3 out of 5
| Language | : | English |
| File size | : | 12351 KB |
| Screen Reader | : | Supported |
| Print length | : | 144 pages |